
Higher tier only
If you are not sure what the mole or the relative atomic mass (Ar) are or perhaps you are not sure how to calculate the relative formula mass (Mr) of a substance then click here before you read this page.
 To calculate the relative formula mass (Mr ) of a compound you simply add up
the relative atomic masses (Ar) of the elements that make it up.
For example the relative formula mass (Mr) of carbon dioxide (CO2) is found by adding
up the relative atomic masses (Ar) of carbon and
oxygen e.g.
To calculate the relative formula mass (Mr ) of a compound you simply add up
the relative atomic masses (Ar) of the elements that make it up.
For example the relative formula mass (Mr) of carbon dioxide (CO2) is found by adding
up the relative atomic masses (Ar) of carbon and
oxygen e.g.
Ar of carbon =12 Ar of oxygen=16
So the relative formula mass (Mr) of CO2 = (Ar of C) + (2x Ar of oxygen)
Mr= 12 + 32 =44.
Also we can say that the mass of 1 mole is just the Mr expressed in grams. So 1
mole of CO2=44g.
This also means the mass of 6x1023 molecules (that is Avogadro's number of molecules) of carbon dioxide has a mass of 44g.
If 44g of CO2 = 1 mole of CO2, then how many
moles is say 11g of CO2 ?
Well:
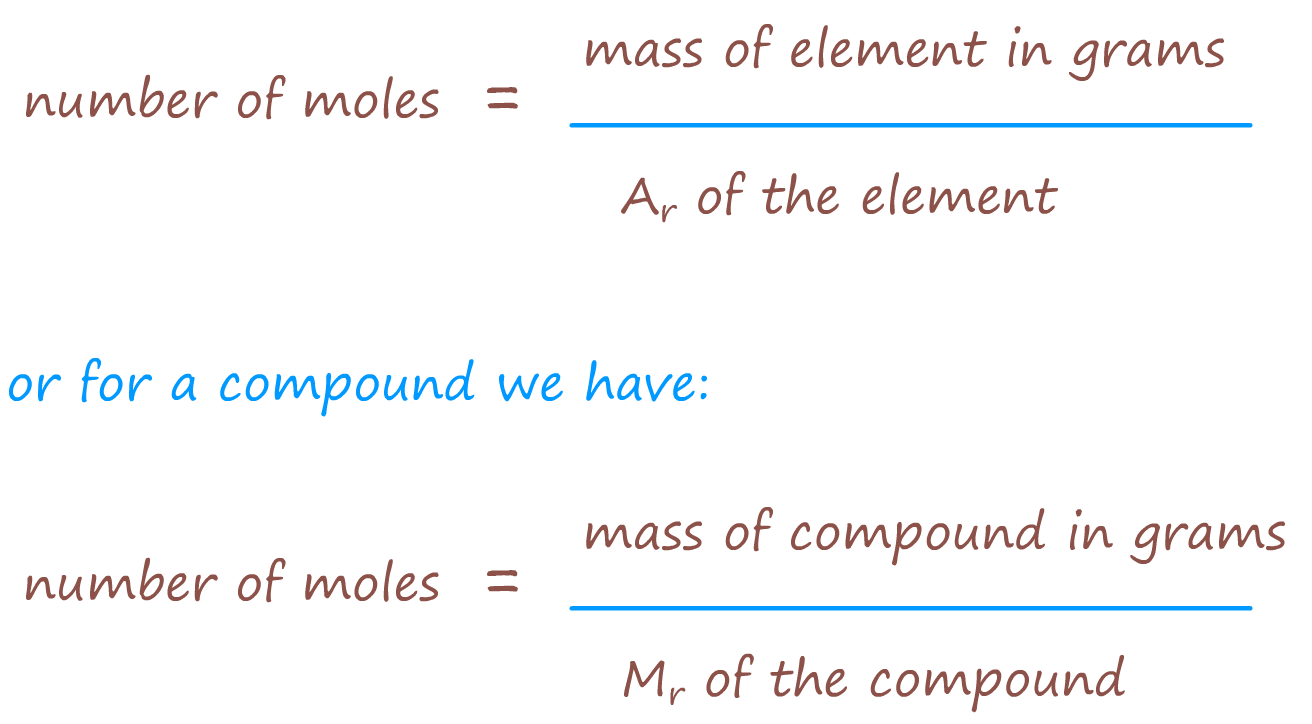 These simple formulae allow you to calculate the number of moles of any element or compound present.
These simple formulae allow you to calculate the number of moles of any element or compound present.

The molecule shown opposite is the sugar glucose; its molecular formula is C6H12O6.
The relative formula mass (Mr) of glucose (C6H12O6) is found by adding
up the relative atomic masses (Ar) of carbon,
hydrogen and oxygen just as we did above for carbon dioxide e.g.
Ar of carbon=12, Ar of hydrogen=1,
Ar of oxygen=16
so Mr of glucose,
C6H12O6 =(6x Ar of C) + (12x Ar of hydrogen) + (6x Ar of oxygen)
Mr= 72 + 12 + 96 =180.
Also the mass of 1 mole is just the Mr expressed in grams. So 1
mole of glucose, C6H12O6 =180g.
So if we had; say 37g of glucose; how many moles of glucose would this be?
Well using the formula above we have:
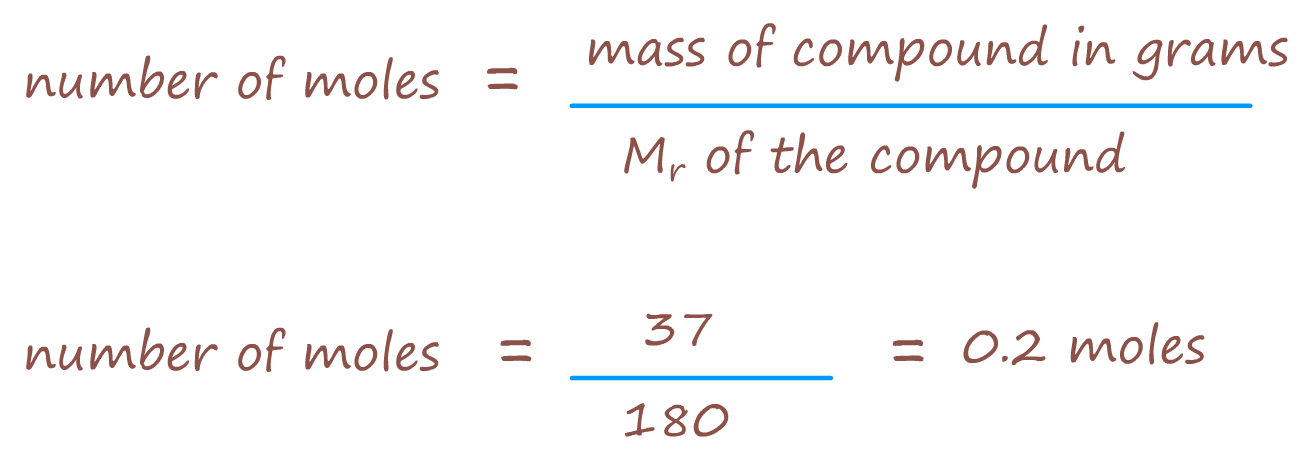
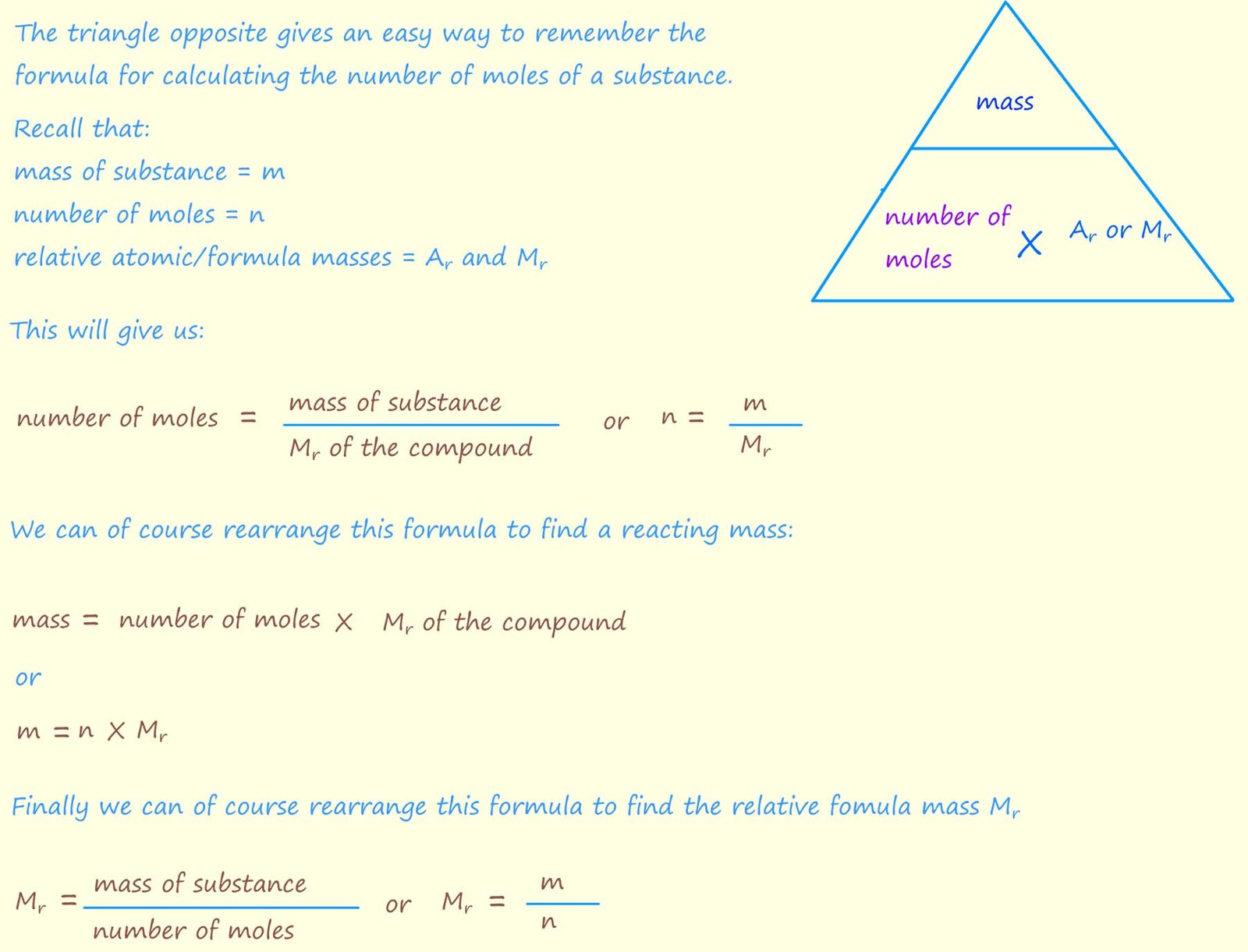
In mole calculations:
You should be able to use the formula below to calculate either:
All that is needed is a bit of simple arithmetic and the ability to rearrange any formula given. The highlighted box below shows all three formulae which you could be asked to use.
What is the mass of 0.65 moles of glucose? To calculate the mass we use the formula :
mass = number of moles x Mr
m= n x Mr
m = 0.65 mol x 180 = 117g
so 0.65 moles of glucose has a mass of 117g.